Introduction: DARA, a human IgGκ monoclonal antibody targeting CD38, is approved as monotherapy and in combination with standard-of-care regimens for relapsed/refractory multiple myeloma and NDMM. In the primary analysis of the phase 2 GRIFFIN study (NCT02874742) in patients with transplant-eligible NDMM, DARA plus RVd (D-RVd) significantly improved rates of stringent complete response (sCR) by the end of post-transplant consolidation therapy versus RVd (Voorhees P, Blood 2020). Here, we present updated efficacy and safety results following 12 months of maintenance therapy with lenalidomide (R) or DARA plus R (D-R).
Methods: Patients with NDMM eligible for high-dose therapy (HDT) and autologous stem cell transplant (ASCT) were randomized 1:1 to RVd ± DARA, stratified by ISS stage and creatinine clearance rate. Patients received 4 induction cycles, HDT, ASCT, 2 consolidation cycles, and maintenance with R ± DARA for 24 months. During induction and consolidation, patients received R 25 mg PO on Days 1‐14; V 1.3 mg/m2 SC on Days 1, 4, 8, and 11; and d 40 mg QW every 21 days. DARA 16 mg/kg IV was given on Days 1, 8, and 15 of Cycles 1‐4 and Day 1 of Cycles 5‐6. During maintenance (Cycles 7-32), patients received R 10 mg (15 mg in Cycles 10+ if tolerated) on Days 1‐21 every 28 days ± DARA 16 mg/kg IV Q8W (or Q4W per patient decision after Amendment 2). The primary endpoint was rate of sCR at the end of post-ASCT consolidation per IMWG criteria, evaluated by a validated computer algorithm. Key secondary endpoints included progression-free survival (PFS) and rate of minimal residual disease (MRD) negativity (10-5 threshold per IMWG criteria) assessed by next-generation sequencing (clonoSEQ; Adaptive Biotechnologies). The primary hypothesis was tested at a 1-sided alpha of 0.10. All secondary analyses were evaluated using a 2-sided P value (alpha 0.05) and were not adjusted for multiplicity.
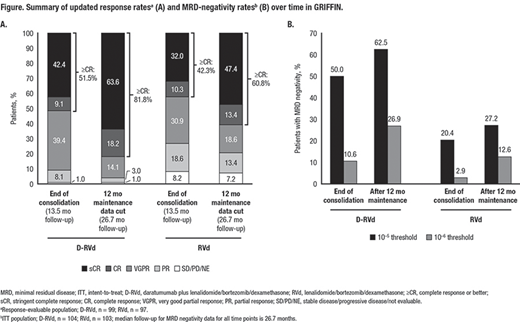
Results: In total, 207 patients were randomized (D-RVd, n=104; RVd, n=103). Baseline demographics and disease characteristics were well balanced between arms. At the end of post-transplant consolidation (median follow-up, 13.5 months) in the response-evaluable population, the sCR rate favored D-RVd versus RVd (42.4% [42/99] vs 32.0% [31/97]; 1-sided P=0.0680). With additional D-R or R maintenance therapy, responses continued to deepen and remained higher for the D-RVd group versus the RVd group. At the 12-months-of-maintenance therapy data cut (median follow-up, 26.7 months), the sCR rate still favored D-RVd versus RVd (63.6% [63/99] vs 47.4% [46/97], 2-sided P=0.0253; Figure). MRD-negativity (10‒5) rates in the ITT population favored D-RVd versus RVd (62.5% [65/104] vs 27.2% [28/103], P<0.0001; Figure), as well as among patients who achieved complete response (CR) or better at that time (76.5% [62/81] vs 42.4% [25/59], P<0.0001). Similarly, MRD-negativity (10‒6) rates favored D-RVd versus RVd in the ITT population (26.9% [28/104] vs 12.6% [13/103], P=0.0140; Figure), as well as among patients who achieved CR or better at that time (34.6% [28/81] vs 18.6% [11/59], P=0.0555). Estimated 24-month PFS rates were 94.5% and 90.8% for the D-RVd and RVd groups, respectively. In total, 14 deaths occurred (n=7 per group), and 9 were due to progressive disease (D-RVd, n=5; RVd, n=4). With longer follow-up, no new safety concerns were observed. 84.8% (84/99) of patients in the D-RVd group and 79.4% (81/102) in the RVd group had grade 3/4 treatment-emergent adverse events (TEAEs). One grade 5 TEAE occurred in the RVd group, which was unrelated to study therapy (unknown cause). Infusion-related reactions occurred in 43.4% (43/99) of patients, with the majority being grade 1 or 2 and occurring in the first cycle.
Conclusions: After 26.7 months of median follow-up, the addition of DARA to RVd induction and consolidation, followed by D-R maintenance in patients with transplant-eligible NDMM continued to demonstrate deep and improved responses, including higher sCR and MRD negativity rates, compared with lenalidomide alone. Maintenance therapy increased sCR and MRD negativity rates, compared to post-consolidation rates. No new safety concerns were observed with longer follow-up.
Support: Alliance Foundation Trials; https://acknowledgments.alliancefound.org; Janssen Oncology
Kaufman:Tecnopharma: Consultancy, Honoraria; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria; Sanofi/Genyzme: Consultancy, Honoraria; AbbVie: Consultancy; Amgen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Honoraria; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria; Pharmacyclics: Membership on an entity's Board of Directors or advisory committees. Sborov:University of Utah: Current Employment; Celgene, Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Personal fees. Reeves:Incyte: Honoraria; Takeda: Honoraria; Bristol Myers Squibb: Speakers Bureau. Rodriguez:BMS, Takeda, Amgen: Consultancy, Speakers Bureau. Chari:Janssen, Celgene, Novartis, Amgen, Bristol-Myers Squibb, Karyopharm, Sanofi, Genzyme, Seattle Genetics, Oncopeptides, Millennium/Takeda, Antengene, Glaxo Smith Kline, Secura Bio: Consultancy; Janssen, Celgene, Novartis, Amgen, Pharmacyclics, Seattle Genetics, Millennium/Takeda: Research Funding. Silbermann:Karyopharm: Consultancy; Janssen: Consultancy; Sanofi-Aventis: Consultancy, Research Funding. Costa:AbbVie: Consultancy; Sanofi: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Genentech: Consultancy; BMS: Consultancy, Honoraria. Anderson:Amgen: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Karyopharm: Consultancy, Honoraria, Research Funding. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, BMS, CareDx, Kite, Karyopharm: Consultancy; BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar: Research Funding. Efebera:Pharmacyclics: Research Funding; Ohio State University: Current Employment; Celgene: Research Funding; Takeda: Honoraria, Speakers Bureau. Holstein:Sorrento: Consultancy; Adaptive Biotechnologies: Consultancy; Takeda: Consultancy; GSK: Consultancy; Celgene: Consultancy; Genentech: Consultancy; Sanofi: Consultancy; Oncopeptides: Consultancy, Research Funding. Costello:Takeda, Celgene: Consultancy, Honoraria. Jakubowiak:Adaptive, Juno: Consultancy, Honoraria; AbbVie, Amgen, BMS/Celgene, GSK, Janssen, Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wildes:Seattle Genetics: Consultancy; Carevive Systems: Consultancy; Janssen: Research Funding. Orlowski:Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties; STATinMED Research: Consultancy; Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding. Shain:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Honoraria, Speakers Bureau; AbbVie: Research Funding; GlaxoSmithKline: Speakers Bureau; Adaptive: Consultancy, Honoraria; Sanofi/Genzyme: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Karyopharm: Research Funding, Speakers Bureau; Amgen: Speakers Bureau. Cowan:Nektar: Research Funding; Janssen: Consultancy, Research Funding; Abbvie: Research Funding; Bristol-Myer Squibb: Research Funding; Celgene: Consultancy, Research Funding; Cellectar: Consultancy; Sanofi-Aventis: Consultancy. Lutska:Janssen: Current Employment. Bobba:Janssen: Current Employment. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Ukropec:Janssen: Current Employment, Current equity holder in publicly-traded company. Vermeulen:Janssen: Current Employment, Current equity holder in publicly-traded company. Lin:Janssen Scientific Affairs: Current Employment, Current equity holder in publicly-traded company. Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding. Voorhees:TeneoBio: Other: Advisory Board; Oncopeptides: Consultancy, Honoraria; Novartis: Consultancy; Janssen: Other: Advisory Board; GSK: Honoraria; BMS: Other: Advisory Board; Adaptive Biotechnologies: Other: Advisory Board.
The specific regimen combination is not yet approved, but individual components are.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal